Many believe only acidic environments cause corrosion, but that’s a misconception. Neutral or alkaline pH levels can also lead to material deterioration, especially if environmental factors like contaminants or fluctuating pH are present. Corrosion depends on more than just pH—it’s affected by conditions like oxygen, ions, and material type. Understanding these myths and facts helps you protect your surfaces better; discovering more will give you a clearer picture of effective corrosion prevention.
Key Takeaways
- Corrosion can occur in neutral or alkaline environments; acidity isn’t the sole factor.
- pH fluctuations and environmental conditions significantly influence corrosion rates beyond just pH level.
- Proper pH measurement with calibrated tools is essential for accurate corrosion risk assessment.
- Material resistance varies; plastics and stainless steel often withstand a wider pH range better.
- Myths about pH being the only corrosion factor lead to underestimating other environmental influences.
What Exactly Is Ph and How Is It Measured?

Have you ever wondered what pH really measures? The pH scale is a way to determine how acidic or alkaline a solution is, ranging from 0 to 14. To find out a solution’s pH, scientists use measurement techniques like pH meters or indicator strips. A pH meter works by detecting hydrogen ion activity in the liquid, providing precise readings. Indicator strips change color based on the solution’s pH, giving a quick visual cue. Understanding these measurement techniques is essential because pH levels influence many chemical processes, including corrosion. Whether for industrial applications or scientific experiments, accurately measuring pH helps you assess the environment’s acidity or alkalinity. This knowledge is fundamental before exploring how pH affects corrosion and material durability.
The Myth That Only Acidic Solutions Cause Corrosion

Many people believe that only acidic solutions cause corrosion, but this is a common misconception. While acidic environments are known to accelerate corrosion, materials aren’t immune in neutral or even slightly alkaline conditions. Corrosion can occur in various pH levels, especially if other factors like moisture, salts, or pollutants are present. It’s a myth that only acids damage metals; in reality, neutral or alkaline environments can also cause deterioration, depending on the material’s neutrality and exposure conditions. Recognizing that corrosion isn’t limited to acidic solutions helps you better understand how different environments impact material durability. This awareness is *essential* for selecting appropriate protective measures, regardless of whether the environment is acidic, neutral, or alkaline. Additionally, understanding the environmental factors that influence corrosion can aid in developing more effective prevention strategies.
How Alkaline Environments Influence Material Durability

Alkaline environments can markedly impact the durability of materials, even though they are often considered less aggressive than acidic conditions. In such environments, your materials’ resilience depends on their chemical composition and protective coatings. Here are some key points:
Alkaline environments significantly influence material durability through chemical composition and protective coatings.
- Corrosion Rates: Alkaline conditions may slow corrosion for some metals but accelerate it for others, affecting longevity.
- Material Compatibility: Not all materials withstand high pH levels equally, so choosing corrosion-resistant alloys is essential.
- Surface Stability: Alkaline environments can promote the formation of protective oxide layers, enhancing durability.
- Maintenance Needs: Regular inspections and proper material selection help maintain resilience in alkaline settings.
- Understanding the corrosion mechanisms in alkaline environments is crucial for selecting appropriate materials and protective strategies.
Understanding how alkaline environments influence material resilience allows you to better protect structures and prolong their lifespan.
Common Misconceptions About Neutral Ph and Corrosion Risk

Contrary to popular belief, a neutral pH of 7 does not guarantee immunity from corrosion risks. Many assume that staying within the neutral zone on the pH scale means your materials are safe, but that’s a misconception. Corrosion prevention depends on more than just pH; factors like contaminants, oxygen, and chemical composition also influence risk. Even solutions with a neutral pH can cause corrosion if they contain aggressive ions or pollutants. Understanding that pH alone isn’t the full story helps you better assess material vulnerability. Relying solely on neutral pH as a safeguard can lead to overlooked corrosion threats. To effectively protect your assets, consider exhaustive corrosion prevention strategies that account for pH fluctuations and other environmental factors, not just the pH level. Additionally, Honda Tuning knowledge highlights that material choices and protective coatings play crucial roles in corrosion resistance, regardless of pH.
The Role of Ph Fluctuations in Metal Corrosion Processes

While maintaining a neutral pH level is important, fluctuations in pH can considerably accelerate metal corrosion. Sudden changes in pH reduce pH stability, making metals more vulnerable. These fluctuations can cause localized corrosion, especially when pH drops or rises sharply. To combat this, corrosion inhibitors are often used to buffer pH levels and protect surfaces. Here’s what you should know:
- pH fluctuations disrupt the protective oxide layers on metals.
- Rapid pH changes increase corrosion rates markedly.
- pH stability helps maintain the effectiveness of corrosion inhibitors.
- Consistent monitoring can prevent damaging pH swings, extending equipment lifespan.
- Monitoring air quality is essential in environments where pH fluctuations pose a risk to metal surfaces.
Understanding these factors helps you manage corrosion risks effectively and choose the right corrosion inhibitors for your environment.
Why Ph Alone Doesn’t Determine Corrosion Severity
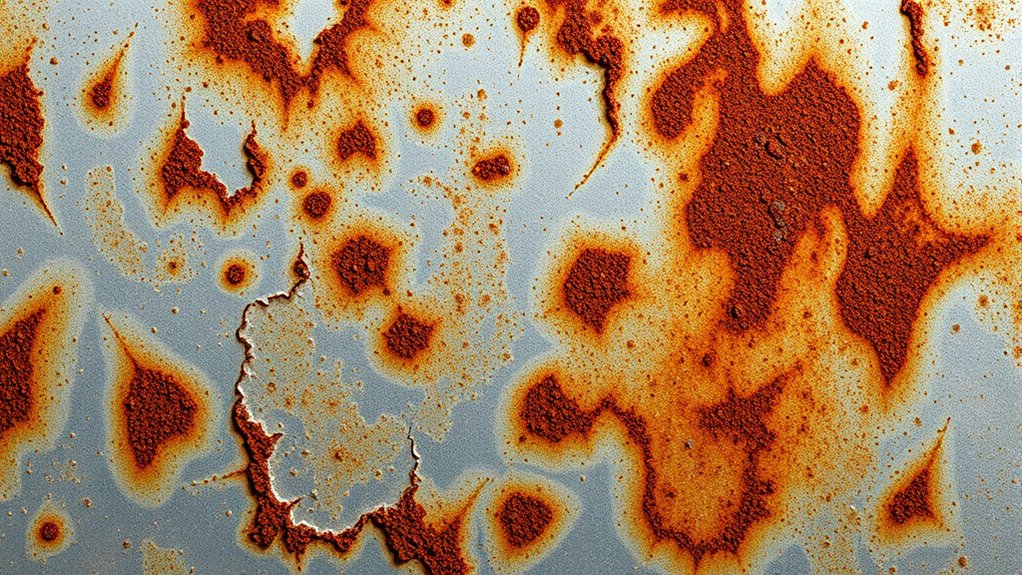
pH level is a key factor in corrosion, but it doesn’t tell the whole story. While a low pH can accelerate corrosion, other elements like pH buffering systems help maintain stability, reducing risk. Corrosion inhibitors play a vital role by forming protective layers on metal surfaces, regardless of pH fluctuations. These inhibitors can neutralize aggressive ions or create barriers that slow the corrosion process. Relying solely on pH measurements oversimplifies the picture; corrosion severity depends on multiple factors, including the presence of contaminants, metal type, and environmental conditions. Understanding this interplay helps you better assess corrosion risks and choose appropriate treatment strategies, rather than focusing only on pH levels. Additionally, corrosion mechanisms are complex processes influenced by various environmental and chemical factors, making comprehensive analysis essential.
Corrosion Protection Strategies That Really Work

Effective corrosion protection relies on a combination of proven strategies rather than just monitoring pH levels. You need to actively manage chemical reactions to prevent metal deterioration. Protective coatings form a barrier, stopping corrosive agents from reaching metal surfaces. Here are four strategies that really work:
- Apply high-quality protective coatings to shield metals from moisture and chemicals.
- Use corrosion inhibitors that interfere with chemical reactions responsible for corrosion.
- Implement cathodic protection systems to control electrochemical reactions.
- Ensure proper maintenance and regular inspections to catch early signs of corrosion.
Color accuracy and proper contrast management further enhance visual clarity and image quality in protective environments.
The Impact of Ph on Different Types of Materials

Understanding how pH levels affect different materials is essential for preventing corrosion and ensuring longevity. The material’s compatibility with pH varies, and environmental factors like moisture, temperature, and pollutants influence corrosion risk. Acidic (low pH) environments accelerate metal deterioration, especially with carbon steel and aluminum, while alkaline (high pH) conditions may cause concrete deterioration. Some plastics and stainless steel resist pH changes better, making them suitable in harsh conditions. Here’s a quick overview:
| Material Type | pH Impact |
|---|---|
| Metals (Steel, Aluminum) | Corrode faster in acidic environments |
| Concrete | Deteriorates under high pH or acidic conditions |
| Plastics/Stainless Steel | More resistant, suitable for varied pH |
Knowing this helps you choose the right material for your environment, especially considering material resistance to different pH levels.
How to Properly Test and Interpret Ph Levels for Corrosion Prevention

To prevent corrosion effectively, you need to accurately test and interpret pH levels in your environment. Proper pH monitoring helps you identify when conditions favor corrosion and when to apply corrosion inhibitors. Here’s how to do it:
- Use a calibrated pH meter or pH test strips for precise measurements.
- Collect samples from different areas to get an accurate overview.
- Record your readings consistently to track pH changes over time.
- Interpret the results by understanding that a pH below 7 indicates acidity, increasing corrosion risk, while above 7 suggests alkalinity, which may require specific inhibitors.
- Recognizing the importance of pH monitoring in corrosion prevention can help you maintain the integrity of your materials.
Frequently Asked Questions
How Do Different Environmental Factors Influence Corrosion Beyond Ph?
Environmental factors like humidity, temperature, and pollutants vitally impact corrosion beyond pH. High humidity accelerates moisture buildup, speeding up oxidation. Elevated temperatures increase reaction rates, while pollutants like salt or sulfur compounds promote aggressive corrosion. You should consider environmental chemistry when choosing materials, opting for corrosion-resistant options. Proper material selection helps mitigate these effects, ensuring longevity. Regular maintenance and protective coatings are also essential to combat environmental influences and extend your assets’ lifespan.
Can Ph Levels Change Over Time in the Same Environment?
Is the environment’s pH level a static fortress? Not quite. Over time, pH levels can shift due to environmental buffering, weather changes, and chemical reactions, making the pH stability a moving target. You should monitor these fluctuations because they directly influence corrosion rates. Changes in pH can accelerate or inhibit corrosion, so understanding these dynamics helps you better protect your materials from unexpected deterioration.
What Are the Best Practices for Monitoring Ph in Industrial Settings?
You should regularly perform pH measurement techniques using calibrated sensors to guarantee accurate readings. Start by calibrating your sensors with standard buffer solutions before each use, and check calibration periodically during monitoring. Keep sensors clean and properly stored when not in use. Using reliable equipment and consistent calibration routines helps you detect pH changes early, preventing corrosion issues and maintaining ideal process conditions in your industrial environment.
How Do Coatings and Inhibitors Interact With Ph to Prevent Corrosion?
Imagine your machinery’s life depends on it—coatings and inhibitors work together, yet their effectiveness hinges on pH compatibility. When coatings are compatible, they form a barrier that shields against corrosion, while inhibitors react with the environment to neutralize corrosive agents. Proper pH levels guarantee inhibitors remain active, maximizing their effectiveness. By understanding this interaction, you can prevent corrosion and extend equipment life, saving costs and avoiding unexpected failures.
Are Some Materials More Resistant to Ph Fluctuations Than Others?
Yes, some materials are more resistant to pH fluctuations due to their superior material durability and pH stability. For example, stainless steel and certain plastics maintain their integrity better when pH levels change, reducing corrosion risk. You should choose materials with proven pH stability for environments with variable pH, as they offer enhanced durability and longevity, helping you prevent costly corrosion-related failures.
Conclusion
Understanding pH and its role in corrosion helps you make better decisions to protect your materials. For example, if you ignore pH fluctuations in a water system, you might experience unexpected metal deterioration, costing you time and money. By testing and adjusting pH levels appropriately, you can prevent costly damage and extend your equipment’s lifespan. Remember, misconceptions can lead to overlooked risks—staying informed is your best defense against corrosion.









